Making Progress in Cancer Technology
OmniNano is a new combination therapy that uses nano-technology to fight pancreatic and liver cancers in the body. This innovative approach has given doctors and scientists a new outlook for the future of cancer technology.
Let us show you how together we can make a difference in delivering a better cancer treatment for future generations.
Cancer Statistics
Future Expectations
PDAC is expected to become the second leading cause of cancer-related deaths by 2030.
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive cancer with poor prognosis statistics based on the lack of early detection. PDAC accounts for more than 90% of pancreatic cancer cases.
Future Expectations
HCC is expected to become the third leading cause of cancer-related deaths by 2030.
Hepatocellular carcinoma (HCC) is the most common type of liver cancer, making up more than 80% of cases in the United States. HCC has a poor prognosis and is typically diagnosed late in its course.
The Challenges
Tumor Heterogeneity
A major challenge presented when finding treatment for these cancers is tumor heterogeneity, or the differences between cancer cells within a single tumor.
Combination Therapy
In order to treat PDAC, Folfirinox or Abraxane and Gemcitabine are administered. For HCC, Atezolizumab and Bevacizumab are used.
Effective Delivery
Our primary goal is to effectively produce a way to deliver the combination therapy and become an accessible option for doctors and patients.

OmniNano's goal is to deliver a powerful and effective therapy for the treatment of pancreatic and liver cancers and become an accessible option for doctors and patients.
The Technology
OmniNano uses a 3rd Generation nanomedicine (M-CPA/PTX) that simultaneously modulates tumor stroma, eliminates tumor cells, and induces differentiation.
The diagram above displays how the nano-drug activates... consectetuer adipiscing elit, sed diam nonummy nibh euismod tincidunt ut laoreet dolore magna aliquam erat volutpat. Ut wisi enim ad minim veniam, quis nostrud exerci tation ullamcorper suscipit lobortis nisl ut aliquip ex ea commodo consequat.
Research Findings
Positive outlook
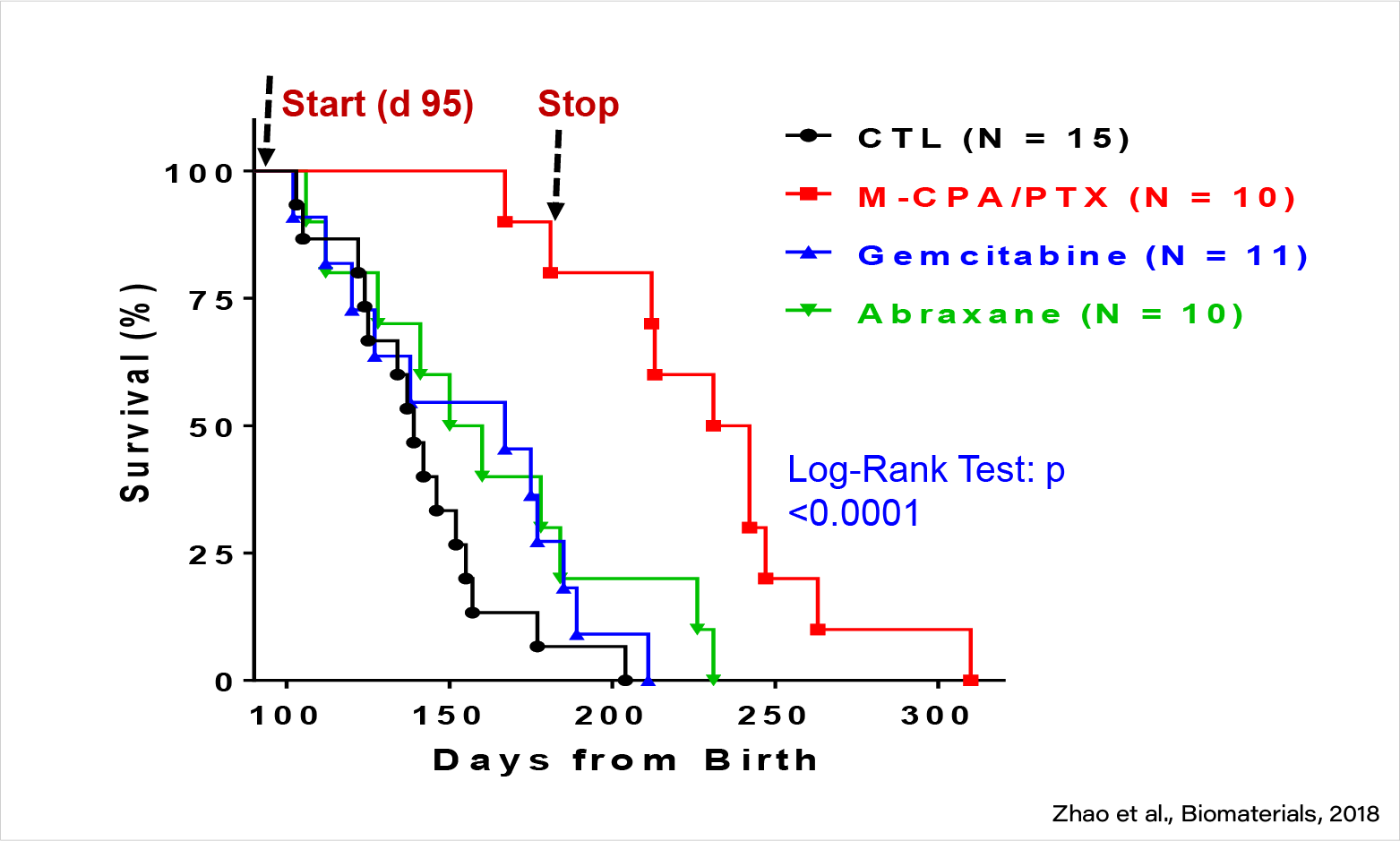
M-CPA/PTX demonstrated remarkable antitumor activity in KPC GEMM of PDAC.
Lorem ipsum dolor sit amet, consectetuer adipiscing elit, sed diam nonummy nibh euismod tincidunt ut laoreet dolore magna aliquam erat volutpat. Ut wisi enim ad minim veniam, quis nostrud exerci tation ullamcorper suscipit.
Positive outlook
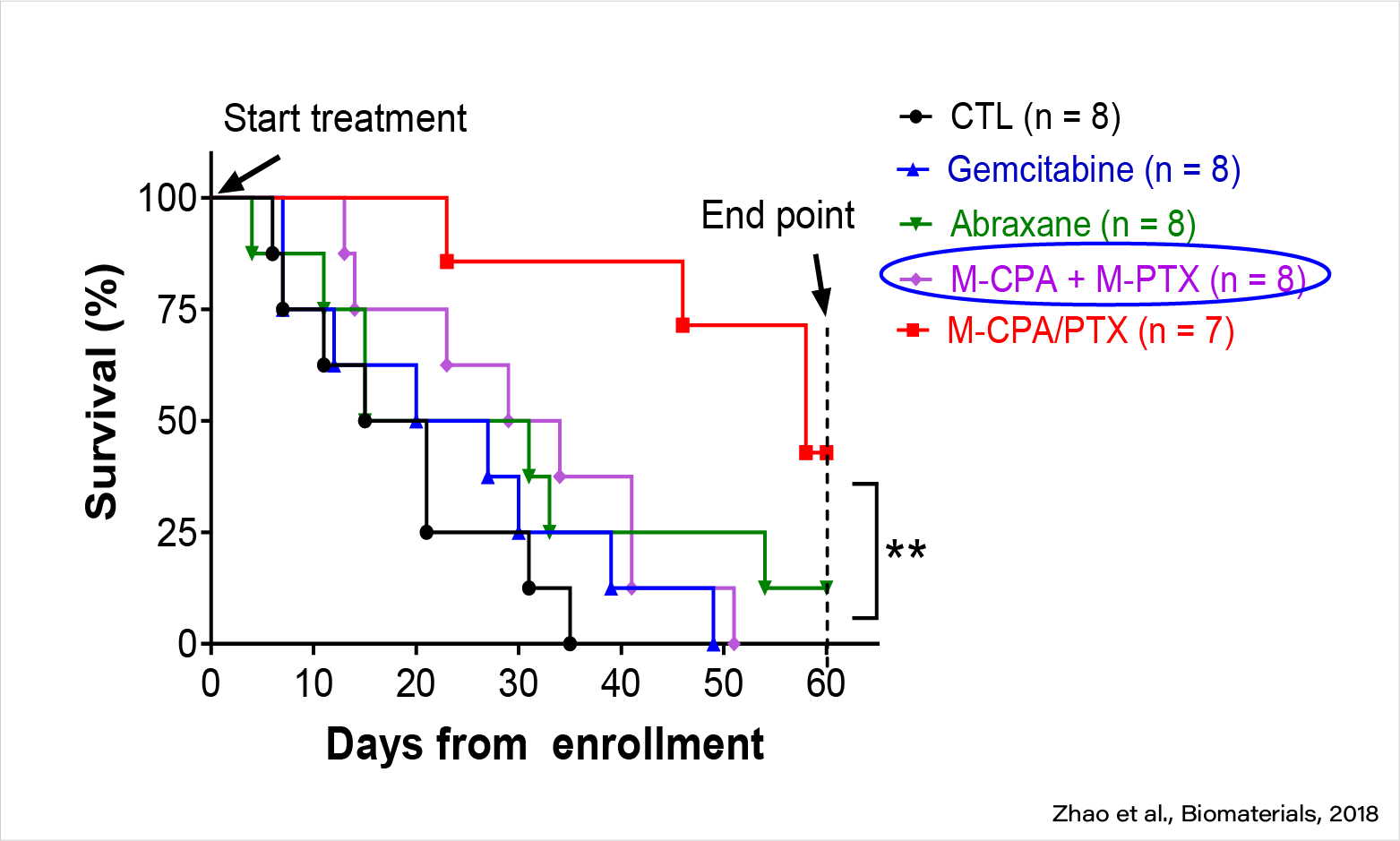
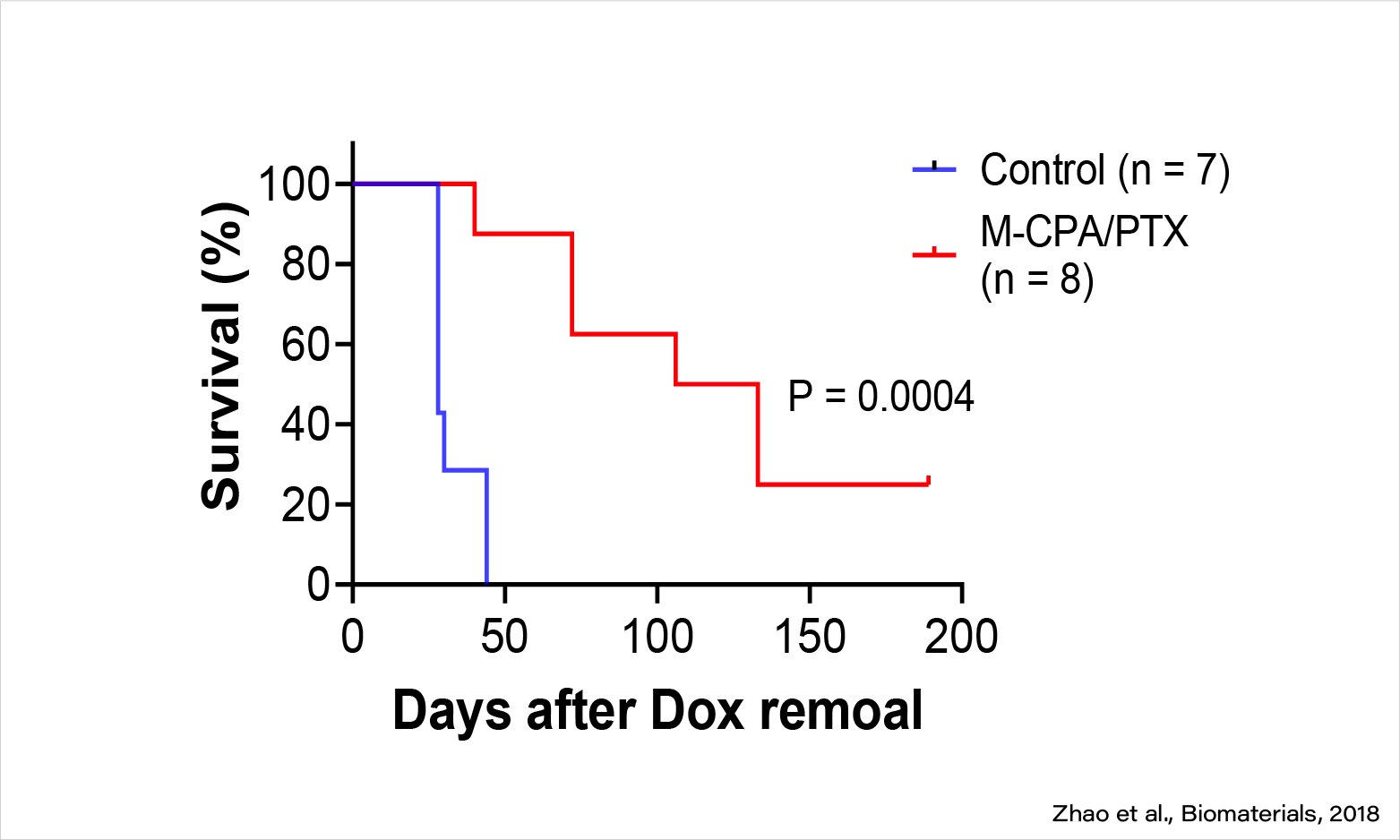
M-CPA/PTX is highly efficacious against PDAC in KPC mice (late treatment).
Lorem ipsum dolor sit amet, consectetuer adipiscing elit, sed diam nonummy nibh euismod tincidunt ut laoreet dolore magna aliquam erat volutpat. Ut wisi enim ad minim veniam, quis nostrud exerci tation ullamcorper suscipit.
Positive Outlook
M-CPA/PTX demonstrated remarkable antitumor activity in c-Myc-driven GEMM of liver cancer.
Lorem ipsum dolor sit amet, consectetuer adipiscing elit, sed diam nonummy nibh euismod tincidunt ut laoreet dolore magna aliquam erat volutpat. Ut wisi enim ad minim veniam, quis nostrud exerci tation ullamcorper suscipit.
Positive outlook
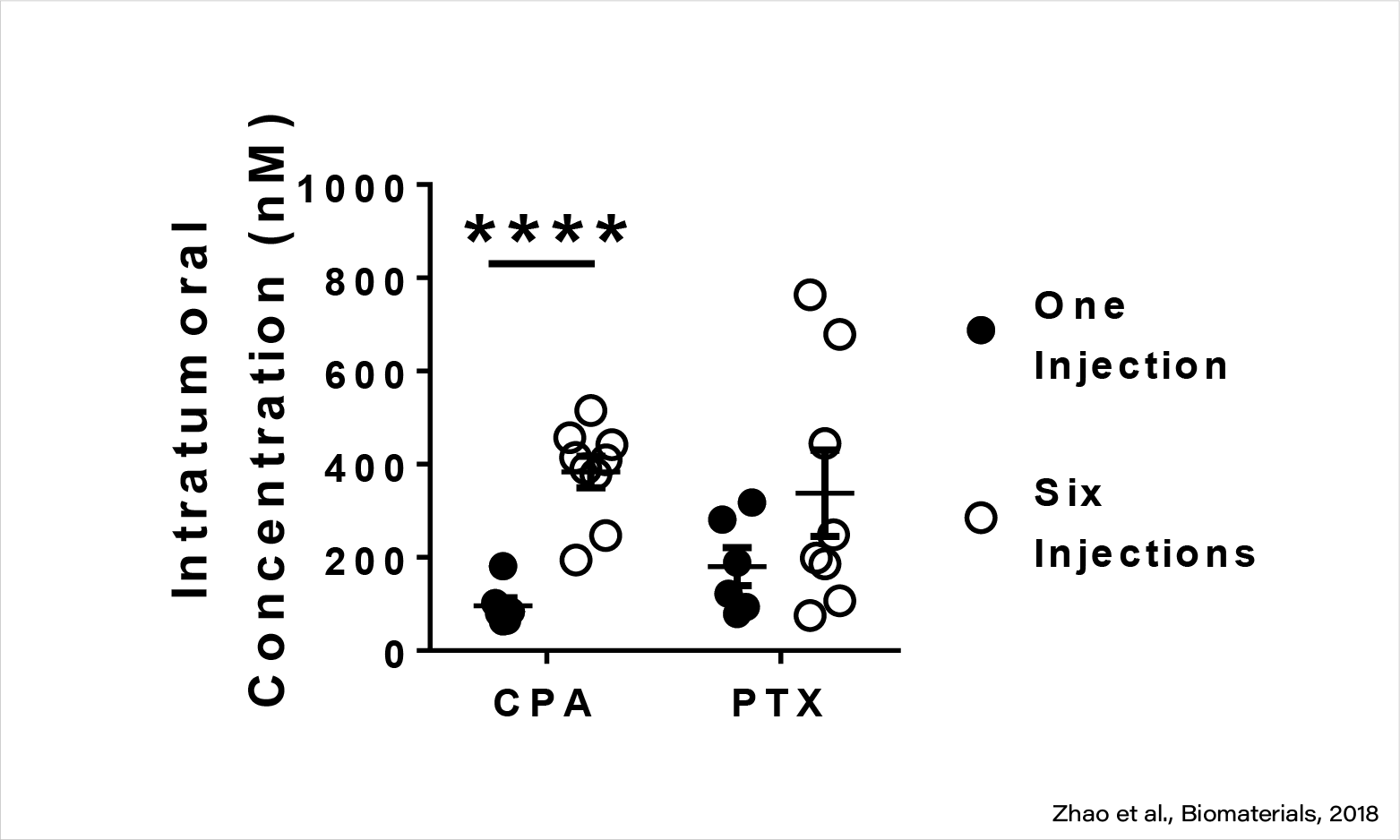
M-CPA/PTX effectively delivered both CPA and PTX to pancreatic cancer In Vivo.
Lorem ipsum dolor sit amet, consectetuer adipiscing elit, sed diam nonummy nibh euismod tincidunt ut laoreet dolore magna aliquam erat volutpat. Ut wisi enim ad minim veniam, quis nostrud exerci tation ullamcorper suscipit.
Therapy Tolerability
M-CPA/PTX was well tolerated in recent research. The images below demonstrate minimal difference in the comparison between the mice control group and the group treated with the combination therapy.
Competitive Landscape
Pancreatic & Liver Cancer Market
Pancreatic Cancer U.S. New Cases in 2021: 60,430
The Global Pancreatic Cancer Therapeutics Market was worth USD 2.6 billion in 2021 and estimated to be growing at a CAGR of 7.54%, to reach USD 3.7 billion by 2026.
Liver Cancer U.S. New Cases in 2021: 42,230
The Liver Cancer Treatment Market was worth USD 2.0 billion in 2020 and is projected to reach USD 7.3 billion by 2028, growing at a CAGR of 17.7% from 2021 to 2028.
Pancreatic & Liver Cancer Competitive Market
Pancreatic Cancer:
There are only 4 FDA approved chemotherapeutics: ABRAXANE, Gemcitabine, 5 FU, and Irinotecan Liposome Injection. In addition, FOLFIRINOX (combination of 5 FU/ Leucovorin, Irinotecan, and Oxaliplatin) is commonly used for metastatic cancer. These treatments provide only a marginal survival benefit over no therapy.
Liver Cancer:
Approved therapeutics include Sorafenib Monotherapy (Rafi/ VEGFRi) which has an OS of 9.2-10.7 months, and Bevacizumab plus Atezolizumab (anti-VEGF + anti-PD-L1) whose OS is better than the Sorafenib Monotherapy.
Competitive Landscape for Nano-Paclitaxel
Protein-bound paclitaxel, also known as nanoparticle albumin-bound paclitaxel or nab-paclitaxel, is an injectable formulation of paclitaxel used to treat breast cancer, lung cancer and pancreatic cancer, among others. It’s delivery vehicle is albumin.